Sulfur Dichloride State At Room Temperature
An idealized but complicated equation is.
Sulfur dichloride state at room temperature. Relative atomic mass the mass of an atom relative to that of. Sulfur dichloride worksites should be equipped with emergency showers and eyewash equipment for this purpose. Is state of matter a physical or a chemical. Pure disulfur dichloride is a yellow liquid that smokes in moist air due to reaction with water.
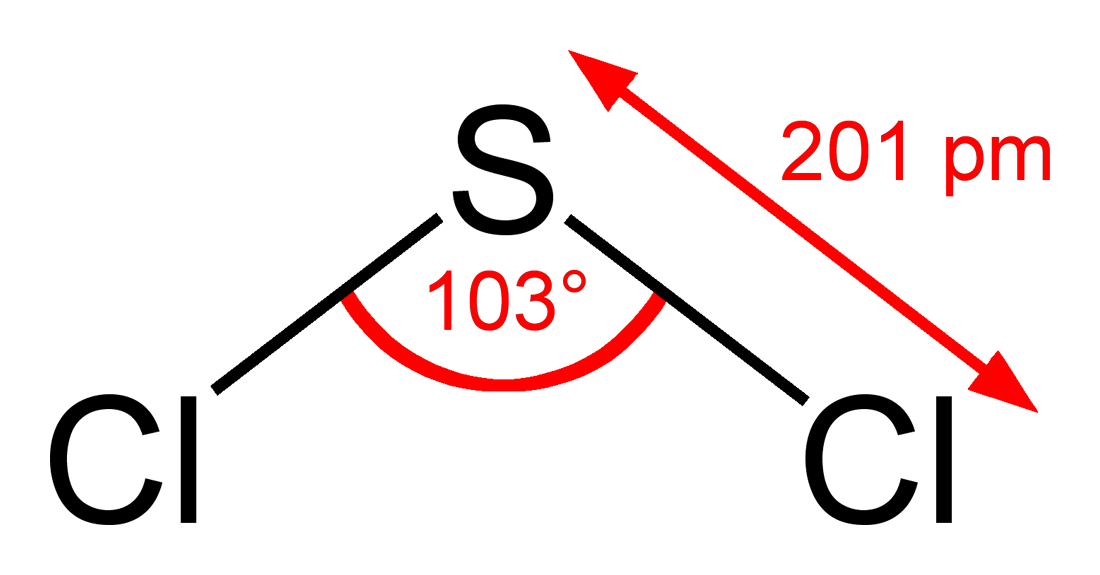
Sulfuryl chloride is commonly confused with thionyl chloride socl 2 the properties of these two sulfur oxychlorides are quite different. High pressure solid allotropes. The pressure temperature p t phase diagram for sulfur is complex see image. Separation of scl 2 from s 2 cl 2 is possible via distillation with pcl 3 to form an azeotrope of 99 purity however sulfur dichloride loses chlorine slowly at room temperature.
The region labeled i a solid region is α sulfur. Sulfuryl chloride is a source of. No it s not a change in the state of matter is a physical change if that s what you were trying to ask. Sulfur dioxide in the air comes mainly from activities such as the burning of coal and oil at power plants or from copper smelting.
Sulfuryl chloride is an inorganic compound with the formula so 2 cl 2 at room temperature it is a colorless liquid with a pungent odor sulfuryl chloride is not found in nature as can be inferred from its rapid hydrolysis. The reaction proceeds at usable rates at room temperature. Sulfur dioxide is a colorless gas with a pungent odor. Sulfur is a solid at room temperature.
In a high pressure study at ambient temperatures four new solid forms termed ii iii iv v have been characterized where α sulfur is form i. Disulfur dichloride s 2 cl 2 is the most common impurity in scl 2. The temperature at which the liquid gas phase change occurs. While sulfur dichloride does not burn easily it may ignite other combustible materials eg wood paper oil.
It is a liquid when under pressure and it dissolves in water very easily. Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase. It is produced by partial chlorination of elemental sulfur. Solid forms ii and iii are polymeric while iv and v are metallic and are.
16 s 2 cl 2 16 h 2 o 8 so 2 32 hcl 3 s 8. Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.